|
|
Part 1 - Engine / Part 2 - Suspensionk / Part 3 - RustRust - the brown pestilenceA reddish brown decomposition product of iron with a flaked consistency, being created in humid, oxygenic environment is on the tip of every 2CV driver's tongue: 'RUST'. Chemically, rust consists of ferrous oxides. Iron and steel are extracted from iron ore (=oxides), hence the natural life cycle of an 2CV end like it started... To retard the disintegration of your loved car, there are some strategies, which should be explained here. ChemistryRusting is chemically the conversion of elemental iron into Iron(III)-hydroxide in the presence of water and oxygen. This process is running in different steps and the total equation looks like this: 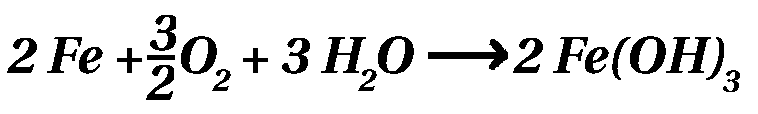 Hydrogen is more noble than iron. Thus all metals (not only iron) being less noble than hydrogen are transferring electrons to hydronium ions, meaning those metals are oxidized. Besides iron the metals lead, zinc, aluminum are also less noble than hydrogen. Tin, copper, gold and platinum are more noble then hydrogen. So why does aluminum not oxidizing? Well, is does, but only at the surface! The aluminum oxide at the surface of the metal shields the elemental aluminum below from the oxygen outside. The iron oxide at the surface is not protecting the elemental iron below, because the iron oxide layer is penetrable by oxygen and water molecules. Hence the iron is still dissolved below the oxide layer. Why is iron covered by a zinc layer? Because zinc is less noble than iron! Hence zinc is disintegrating prior to iron. Furthermore, zinc forms a dense layer of zinc oxide, too (like aluminum does), inhibiting the corrosion of the zinc below. Part 1 - Engine / Part 2 - Suspensionk / Part 3 - Rust |